Electropolishing removes metal from a workpiece by passing an electric current while the workpiece is submerged in an electrolyte of a specific composition. The process is essentially the reverse of electroplating. In a plating system, metal ions are deposited from the solution onto the workpiece. In an electropolishing system, the workpiece itself is eroded, adding metal ions to the solution.
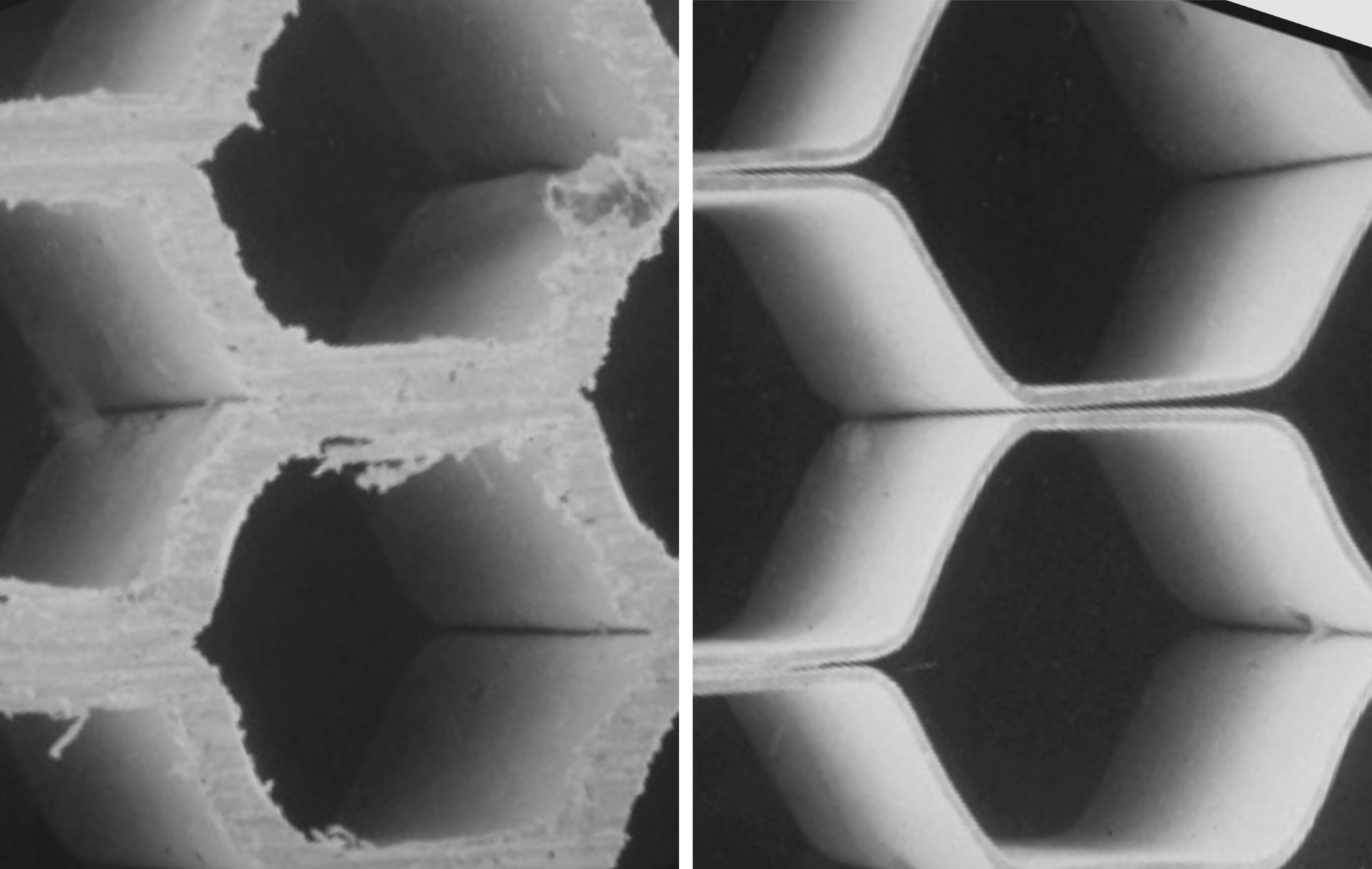
Electropolishing is an efficient method of deburring – even in parts that would be difficult to treat mechanically. Photo: Polygram, Munich (D)
A typical electropolishing installation is similar in appearance to an electroplating line. A power source converts AC current to DC at low voltages. A tank fabricated from plastic or lead-lined tanks is commonly used to hold the chemical bath. A series of lead, copper or stainless steel cathode plates are lowered into the bath, to the negative (-) side of the power source. A part or group of parts is fixed to a rack made of titanium, copper or bronze. That rack in turn is coupled to the positive (+) side of the power source.
The workpiece is thus connected to the positive (anodic) terminal, while the negative (cathodic) terminal is connected to a suit- able conductor. Both positive and negative terminals are submerged in the electrolyte, forming a complete electrical circuit. The current applied is direct (DC) current.
he workpiece being the anode of the electro- chemical process, material is removed from the surface .
As the adjoining illustration shows, the metal part is charged positive (anodic) and immersed in the chemical bath. When current is applied, the electrolyte acts as a conductor (“tool”) to allow metal ions to be removed from the part. While the ions are drawn toward the cathode, the majority of the dissolved metals remain in solution. Some ions are deposited in sludge form on the cathodes, which require regular cleaning for efficiency. Gassing, in the form of oxygen, occurs at the metal surface, furthering the electrolytic action.
The quantity of metal removed from the workpiece is proportional to the current applied, the efficiency of the electrolyte and the exposure time. In the course of electropolishing, burrs and other projections become areas of very high current density and are preferentially eroded. The workpiece process parameters are set to control the amount of metal removed, so dimensional tolerances are maintained.
In the case of stainless steel, the various elements, which make up the alloy, are removed at different rates. Iron and nickel atoms are more easily extracted from the crystal lattice than are chromium atoms. The electropolishing process removes the nickel and the iron preferentially, leaving a surface rich in chromium. This phenomenon accelerates and improves the passivation of electropolished surfaces.
The fact that electropolishing is a non-distortion process is often overlooked. Electropolished parts are neither subject to mechanical nor thermal stress from polishing media, nor are they impinged or tumbled.
The results can be reproduced with a high degree of precision, so components with tight tolerances can also be treated safely .
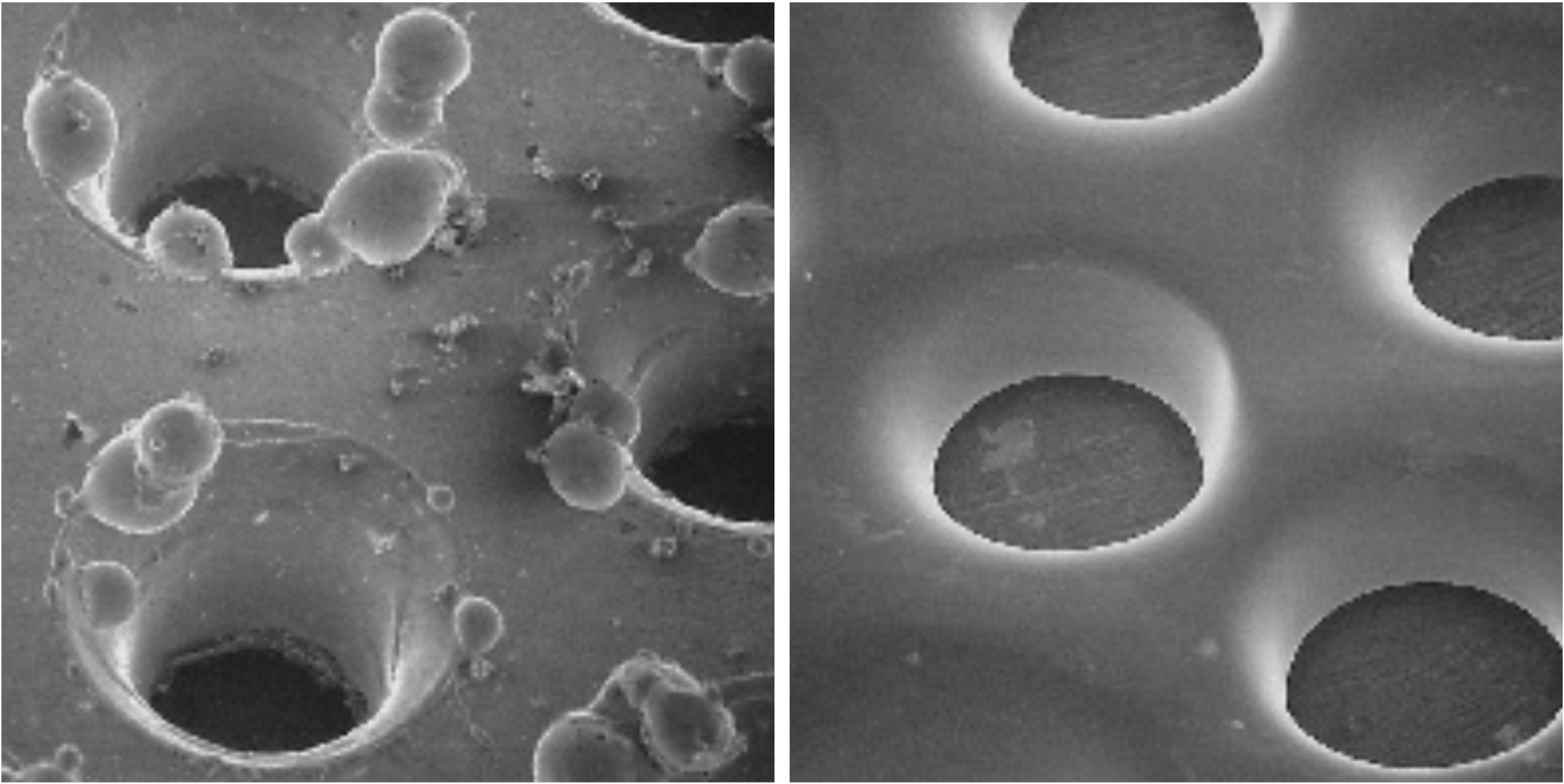
A microscopic view of the same surface before and after electropolishing shows that the process produces clean metallic surfaces. Photo: Polygram, Munich (D)
Given proper process control and procedures, there are no safety hazards, if competent electropolishing contractors are employed who ensure that efficient ventilation systems are used during the process. These operators should also dispose of waste materials, including spent acids, using approved, safe practices.


